Pfizer/BioNTech's novel Covid-19 modified mRNA vaccine was licensed by the FDA in August 2021, on the basis of documentary evidence submitted to the FDA by Pfizer over the preceding 3 months. According to Aaron Siri,
‘During that period, the FDA asserts it conducted an intense, robust, and thorough analysis of those documents to assure the public that the Pfizer vaccine was safe and effective’.
However, the FDA subsequently stonewalled a request to make those documents public, so in September 2021 a group called Public Health and Medical Professionals for Transparency (PHMPT) sued them for the data. PHMPT exists ‘for the sole purpose of disseminating to the public the data and information in the biological product files for each of the COVID-19 vaccines’ and its members include Prof. Allyson Pollock, Dr Iona Heath, Dr Peter Doshi, Dr Tom Jefferson, Prof. Carl Heneghan and Prof. Peter Gøtzsche. It is neither pro-vax or anti-vax.
During the court process, the FDA retaliated and asked a Federal Judge to grant it 55 years to fully release Pfizer’s Covid-19 vaccine data. When pushed, they doubled down and argued they would need 75 years to redact all the personal information in the files. In January 2022, the FDA lost their court battle to hide the data from the public, and were ordered by a federal judge to release 55,000 pages every 30 days until all 450,000 requested pages are public. Given that the FDA decision to approve the Pfizer vaccine had paved the way for vaccine mandates and vaccine passports, this was a huge victory for data transparency in a supposed democracy.
Results in the various vaccine trials were always expressed as Relative Risk Reductions (RRR) which always look more impressive than Absolute Risk Reductions (ARR). For example, Pfizer/BioNTech reported a 95.1% RRR for symptomatic Covid (95% CI, 90.0% to 97.6%) and Moderna a 94.1% RRR (95% CI, 89.1% to 96.8%) for their mRNA-1273 vaccine. The corresponding unreported ARRs from these trial data were 0.7% for the Pfizer/BioNTech and 1.1% f for the Moderna vaccine.
The majority of trial participants were young (only 4% over 75y) and healthy (many people with co-morbidities were excluded) and so not representative of the people who had the most to gain from vaccination. Children, pregnant women, the frail elderly, the immunocompromised, cancer patients, those with autoimmune and chronic inflammatory conditions were specifically excluded, even though Covid-19 gene therapy vaccines would subsequently be recommended for all these groups. The trials were statistically underpowered and therefore not designed to tell us whether the Covid-19 vaccines save lives.
In the published Pfizer trials, we were shown surrogate end-points (reduction in symptomatic Covid, antibody response in children) but no overall mortality benefit. Moreover, a subsequent forensic analysis of the 38 subject deaths in the 6-Month Interim Report revealed a 3.7-fold increase in number of deaths due to cardiovascular events in BNT162b2 vaccinated subjects compared to Placebo controls. Trial researchers judged these events to be unrelated to the gene therapy vaccines.
On 24th January 2024, Mead et al published a peer-reviewed narrative overview of the Covid-19 mRNA vaccines, in which they were highly critical of the handling of the trial data:
‘In hindsight, the previously undisclosed observation that twice as many cardiac deaths occurred proportionately among vaccinated compared to unvaccinated subjects in the Pfizer trial would likely have prompted the FDA’s reevaluation, especially considering the later accumulated data by December 10, 2020, where 17 deaths had occurred.
Delays in documenting these patients’ fatalities in their Case Report File, coupled with the omission of the actual date of death, effectively concealed their deaths during the crucial phase of the EUA approval process, masking the cardiac SAE signal.
In short, the various reporting delays and omissions, if they had been openly discussed and considered by the VRBPAC, might have prolonged the authorization process. The improper reporting and insufficient scrutiny by the VRBPAC may have ultimately enabled Pfizer to manipulate the trial results and obscure the cardiac death signal’.
The Cureus journal’s Editors-in-Chief subsequently retracted this damning report after concerns were raised regarding the validity of some of the cited references and alleged misrepresentation of the data. The authors disagreed with this retraction.
What cannot be contested is that we were given no granular information about the severe adverse events in the vaccine arm of the trials. Judging from testimony given at the expert panel on medical mandates and vaccine injuries in Washington DC, there were trial participants with serious life-changing vaccine adverse events. Worryingly, testimonials suggest that some participants with significant injuries after dose 1 were withdrawn from the trials and their injuries were not described in published reports on vaccine safety and efficacy. This should raise serious questions about bias in the published data.
Peter C Gøtzsche raised this issue in an October 2023 podcast discussion with Professor Christine Stabell Benn (from 30:11). To paraphrase Gøtzsche:
In the Pfizer and Astra Zeneca trials participants were given an app where they could report what they thought were harms but only those harms that the companies had determined beforehand might be of interest and related to the vaccines. So if somebody developed myocarditis on Pfizer’s vaccine or a stroke on the Astra Zeneca vaccine, they couldn’t report it on the app.
One of the participants in the Pfizer trial became severely incapacitated and she dropped out of the trial after the first dose of the vaccine and then she disappeared totally - there was no account of her in the NEJM article so we have to be highly sceptical of what the harms are with the Covid-19 vaccines. We know too little about it.
In the words of Professor David Healy,
‘Patients who drop out of trials because of an adverse event can be designated as having intercurrent illness or some related term. There is no obligation to write a narrative on a patient like this, which leaves anyone who might get to see the records none the wiser as to what has gone on’.
Healy presents the case history of 36 year old Argentine criminal lawyer, Augusto Germán Roux, who had volunteered to participate in the phase 2/3 BioNTech/ Pfizer Covid-19 vaccine clinical trials. Within hours of his second injection in September 2020, Augusto describes feeling unwell with shortness of breath, burning chest pain, extreme fatigue and nausea. For 2 days he remained bed-bound with a high fever and delirium. After collapsing with loss of consciousness he was hospitalised in Buenos Aires, where he was diagnosed with a pericardial effusion, elevated liver enzymes and ‘high probability of adverse reaction to coronavirus vaccine’.
He contacted the trial research team by phone from the hospital to update them. A scan also showed a possible low-grade liver fibrosis but this might not be linked to the vaccine administration. Healy concludes, ‘On a balance of probabilities basis, the most reasonable diagnosis is that after the second vaccine dose Augusto had a vaccine induced pericarditis.’ His liver enzymes remained abnormal even 2 years on.
Augusto’s Clinical Trial Record allegedly stated that his hospital admission was for a bilateral pneumonia and nothing to do with the product of investigation, though his medical records contradict this claim. Against all the evidence (he had tested negative for SARS-CoV-2 infection on PCR and also had negative SARS-Cov2 antibodies), the adverse event was then changed in the trial record to ‘COVID-19 disease’. When Augusto threatened to take the matter to the ANMAT (the Argentinian medicines regulator), the trial’s chief investigator Dr Polack allegedly made a further entry into the event record the day before a hearing was scheduled, stating that Augusto had had ‘an adverse event of severe anxiety starting on September 23 not caused by the vaccine’ - instead he described Augusto as suspecting a conspiracy (between two hospitals) and his anxiety as constitutional.
So not only did the clinical trial record allegedly misrepresent Augusto’s adverse event as one of ‘severe anxiety’ rather than ‘vaccine-induced pericarditis’, but because he withdrew from the study his reaction did not even NEED to feature in the published trial data. 302 subjects dropped out from the vaccine group for ‘protocol deviations’ after dose 2 whereas only 52 on placebo dropped out. Of those vaccine drop-outs, the most commonly cited reason was ‘did not receive 2 vaccinations’ but there is no record of why!
The first trial data would be published in the NEJM on December 10th 2020 (the day before FDA gave EUA for use of this vaccine), with Dr Polack named as first author. Augusto’s injury would not feature in this report.
The PHMPT website serves as a publicly accessible open repository for the court-ordered released Pfizer clinical trial documents as well as the Moderna documents. This facilitates forensic scrutiny by interested public health professionals, medical professionals, scientists and journalists.
One such research volunteer is Dr. Jeyanthi Kunadhasan, an anaesthetist and peri-operative physician in Australia. In a letter to Texas Attorney General Ken Paxton in January 2024, she highlighted two undisclosed deaths of American trial participants (subjects 11141050 and 11201050) in the BNT162b2-vaccinated arm of Pfizer’s clinical trial. These deaths had been reported to the clinical site before the EUA data cut-off date, but in violation of protocol, their recording was delayed until after the data had been submitted to the FDA for EUA approval! If they had been disclosed, it would have shown that the BNT162b2 mRNA COVID vaccine intervention did not reduce deaths.
Kunadhasan concludes:
‘Notably, the omission of the two deaths from the vaccinated arm of the study at this critical juncture of EUA issuance raises substantial concerns about the overall safety reporting of Pfizer’s clinical trial. Patients who volunteered for the clinical trial likely did so, at least in part, in service of humanity. The failure to disclose the patients’ deaths, despite timely notification by loved ones, constitutes a betrayal of their altruism and trust and deserves further investigation’.
The Pressure Group HART (Health Advisory & Recovery Team) also report a number of concerning signals for sudden death overlooked in the Pfizer/BioNTech trial. They cite the case of a 65 year old man (subject 10841470) whose death was categorised as an ‘unvaccinated Covid-19 death’ in the trial findings, when in fact he ‘had received two placebo injections and then got the Moderna vaccine while still in the trial. He developed COVID-19 during the critical post-vaccination period, was hospitalised within a week of receiving the vaccine, and passed away 11 days post-injection.’
In another case, reporting of the sudden death of a 60 year old man (subject 11621327) three days post vaccination was delayed for 70 days, which was coincidentally a week after the ‘data submission deadline that informed the trial's conclusive report leading to its approval’. The cause of death was unrecorded in the report, as no autopsy results were available.
In another article HART draws our attention to the confusing and contradictory data surrounding deaths in the Moderna trial, with deaths disappearing and reappearing in different iterations of data submitted to the FDA for EMA approval.
In November 2021 an 'explosive' article published in the BMJ described how whistleblower Brook Jackson reported her concerns to the FDA about the integrity of the Ventavia’s Pfizer phase 3 trials. She was fired after complaining about Ventavia’s poor research practices. Ms Jackson evidenced lack of blinding, falsification of data, poor monitoring, lack of timely follow-up of patients who experienced adverse events, protocol deviations not being reported, vaccines not stored at proper temperatures, mislabelling of lab specimens and targeting of staff for reporting these types of problems.
The BMJ expose, which raises serious questions about the reliability of trial data upon which the vaccine’s EUA was granted, was ‘fact-checked’ and labelled as ‘misinformation’ by Facebook. This prompted Fiona Godlee, editor of the BMJ, to write an open letter of rebuke to Mark Zuckerberg.
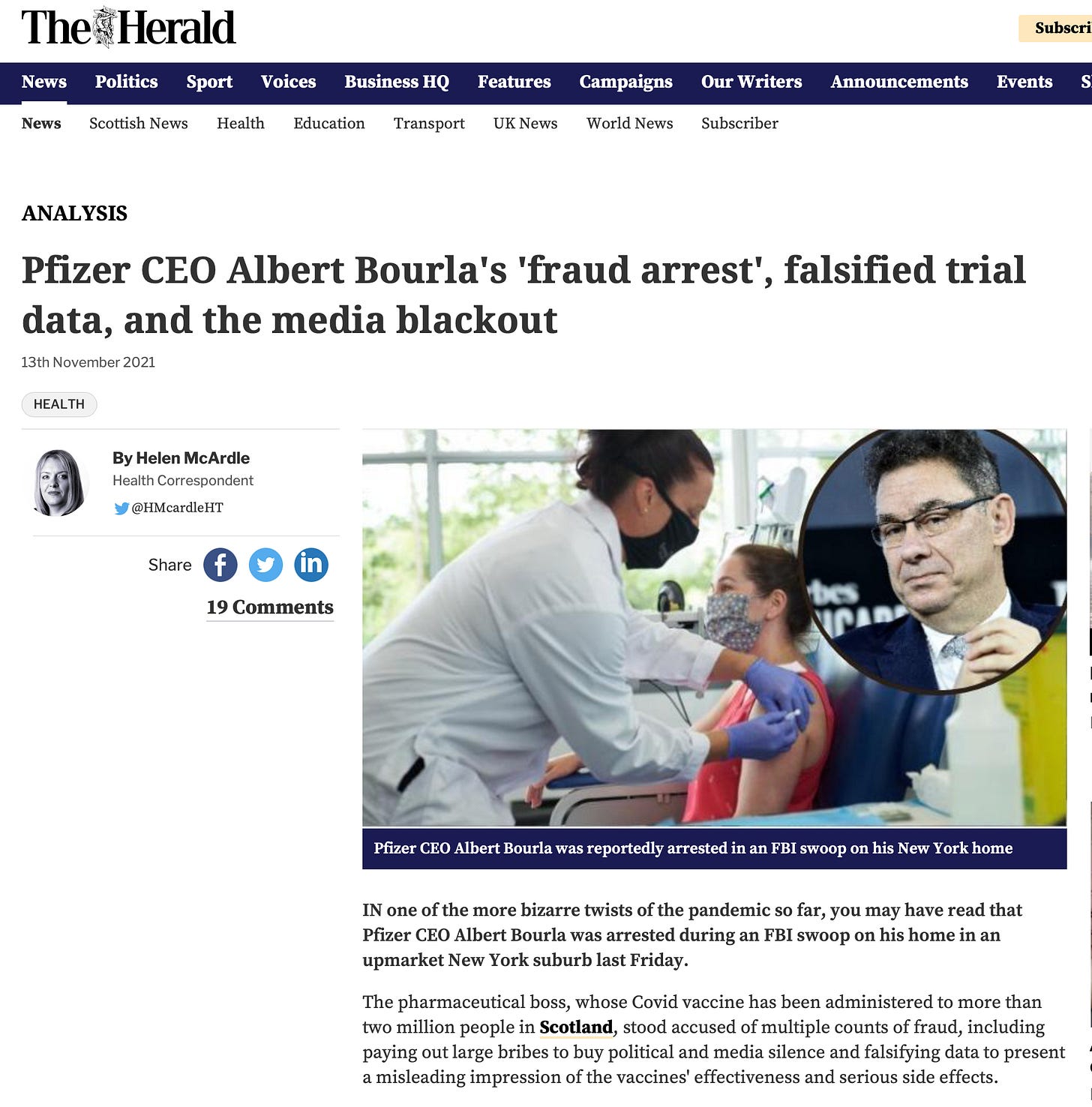
Unsurprisingly, many downplayed the BMJ report’s significance. One Scottish paper created ambivalence and confusion by publishing a bizarre article, wherein the (true) story evidencing ‘Pfizer’s falsifying data to present a misleading impression of the vaccines' effectiveness and serious side effects’ was clumsily interwoven with an outlandish disinformation story about Pfizer CEO Albert Bourla’s FBI arrest for bribery and corruption. Intentional or not, this has all the appearances of a diversionary tactic taken straight out of the Disinformation Playbook strategies.






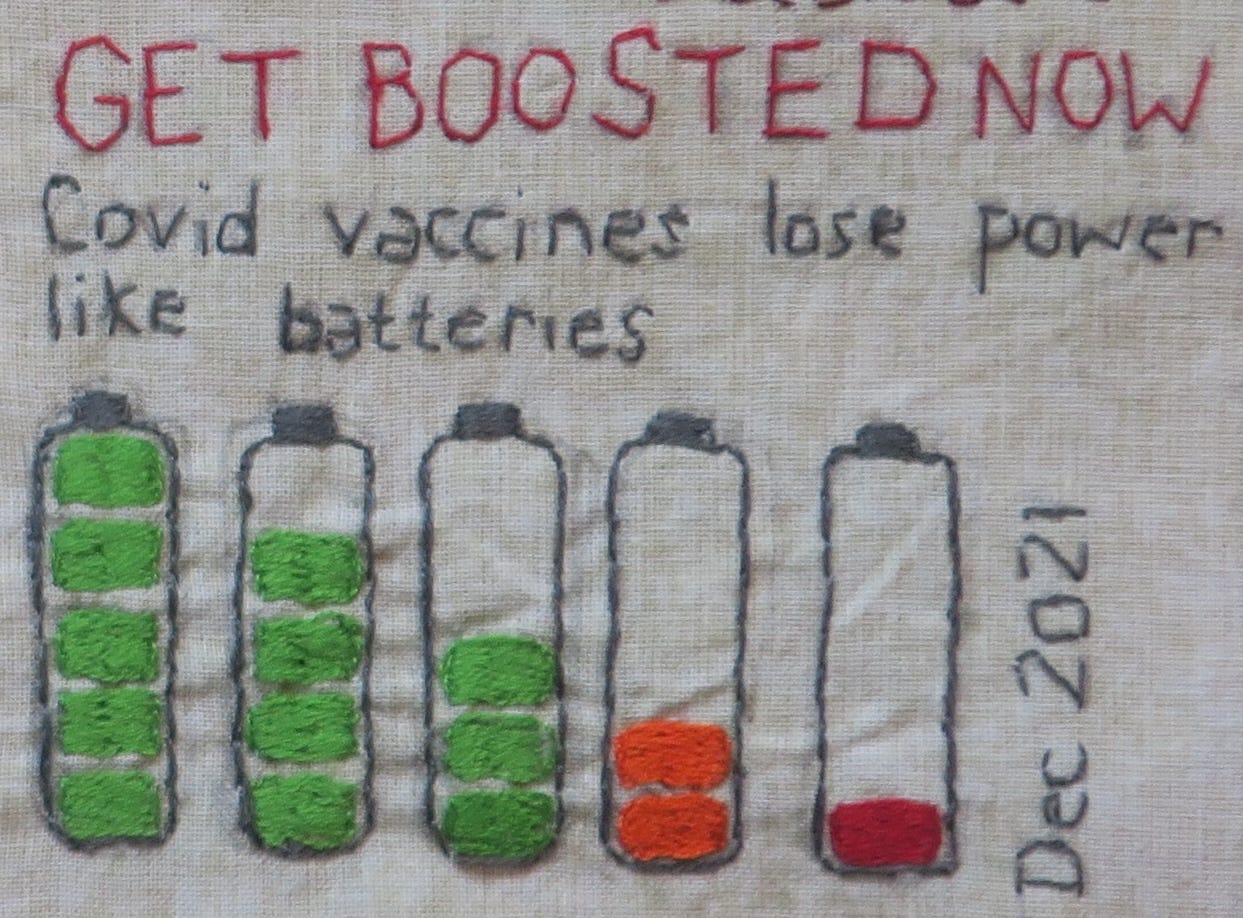





Extremely informative overview. How is such egregious malpractice still largely unknown by the general public? I think we know the answer to that! MSM, Govts., Regulatory bodies and other publicly funded institutions have betrayed their core functions and by association, we, the public. I can never trust them again. Ever!
Very nice summary and I like the embroidered picture of Clare Craig.
Maybe your whole collection could form part of an art installation!
You will find the following paper quite interesting too. It touches on the subject of the problem with the use of leaky vaccines in mass vaccination programs.
CRESSLER, C. E., McLEOD, D. V., ROZINS, C., VAN DEN HOOGEN, J., & DAY, T. (2016). The adaptive evolution of virulence: a review of theoretical predictions and empirical tests. Parasitology, 143(7), 915–930. doi:10.1017/S003118201500092X